Pandemix influenza vaccine (H1N1)
Pandemrix suspension and emulsion for emulsion for injection
Pandemic influenza vaccine (H1N1)(split virion, inactivated, adjuvanted)
PACKAGE LEAFLET: INFORMATION FOR THE USER
For the most up-to-date information please consult the website of the European Medicines Agency (EMEA): http://www.emea.europa.eu/. Read all of this leaflet carefully before you receive this vaccine .
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If any of the side effects gets serious, or if you notice any side effects
not listed in this leaflet, please tell your doctor.
In this leaflet:
1. What Pandemrix is and what it is used for
2. Before you receive Pandemrix
3. How Pandemrix is given
4. Possible side effects
5. How to store Pandemrix
6. Further information
1. What Pandemrix is and what it is used for
- Pandemrix is a vaccine to prevent pandemic influenza (flu).
- Pandemic flu is a type of influenza that occurs every few decades and which spreads rapidly around the world. The symptoms of pandemic flu are similar to those of ordinary flu but may be more severe.
- When a person is given the vaccine, the immune system (the body’s natural defence system) will produce its own protection (antibodies) against the disease. None of the ingredients in the vaccine can cause flu.
2. Before you receive Pandemrix
You should not receive Pandemrix:
• if you have previously had a sudden life-threatening allergic reaction to any ingredient of Pandemrix (these are listed at the end of the leaflet) or to any of the substances that may be present in trace amounts as follows:
egg and chicken protein, ovalbumin, formaldehyde, gentamicin sulphate (antibiotic) or sodium deoxycholate. Signs of an allergic reaction may include itchy skin rash, shortness of breath and swelling of the face or
tongue. However, in a pandemic situation, it may be appropriate for you to have the vaccine provided that appropriate medical treatment is immediately available in case of an allergic reaction.
If you are not sure, talk to your doctor or nurse before having this vaccine.
Take special care with Pandemrix:
• if you have had any allergic reaction other than a sudden lifethreatening allergic reaction to any ingredient contained in the vaccine, to thiomersal, to egg and chicken protein, ovalbumin, formaldehyde, gentamicin sulphate (antibiotic) or to sodium deoxycholate. (see section 6. Further information).
• If you have a severe infection with a high temperature (over 38°C). If this applies to you then your vaccination will usually be postponed until you are feeling better. A minor infection such as a cold should not be a problem, but your doctor or nurse will advise whether you could still be vaccinated with Pandemrix,
• If you are having a blood test to look for evidence of infection with certain viruses. In the first few weeks after vaccination with Pandemrix the results of these tests may not be correct. Tell the doctor requesting these tests that you have recently been given Pandemrix.
In any of these cases, TELL YOUR DOCTOR OR NURSE, as vaccination may not be recommended, or may need to be delayed.
Please inform your doctor or nurse if you have a bleeding problem or
bruise easily.
Taking other medicines
Please tell your doctor or nurse if you are taking or have recently taken any other medicines, including medicines obtained without a prescription or have recently been given any other vaccine.
There is no information on administration of the vaccine Pandemrix with other vaccines. However, if this cannot be avoided, the vaccines should be injected into separate limbs. In such cases, you should be aware that the side effects may be more intense.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant, think you may be pregnant, plan to become pregnant. You should discuss with your doctor whether you should receive Pandemrix.
The vaccine may be used during breast-feeding.
Driving and using machines
Some effects mentioned under section 4. “Possible side effects” may affect the ability to drive or use machines.
Important information about some of the ingredients of Pandemrix
This vaccine contains thiomersal as a preservative and it is possible that you may experience an allergic reaction. Tell your doctor if you have any known allergies.
This medicinal product contains less than 1 mmol sodium (23 mg) and less
than 1 mmol of potassium (39 mg) per dose, i.e. essentially sodium- and potassium-free.
3. How Pandemrix is given
Your doctor or nurse will administer the vaccine in accordance with official recommendations.
The vaccine will be injected into a muscle (usually in the upper arm).
· Adults, including the elderly
A dose (0.5 ml) of the vaccine will be given.
A second dose of 0.5 ml vaccine may be given after an interval of at least three weeks.
· Children and adolescents 10-17 years of age
If it is considered that you need to be vaccinated, you may receive two doses of 0.5 ml vaccine given at least three weeks apart.
· Children 3-9 years of age
If it is considered that your child needs to be vaccinated, he/she may receive one dose of 0.25 ml vaccine and a second dose of 0.25 ml at least three weeks later.
· Children aged from 6 months to 3 years of age
If it is considered that your child needs to be vaccinated, he/she may receive one dose of 0.25 ml vaccine and a second dose of 0.25 ml at least three weeks later.
· Children aged less than 6 months of age
Vaccination is currently not recommended in this age group.
When Pandemrix is given for the first dose, it is recommended that Pandemrix (and not another vaccine against H1N1) be given for the complete vaccination course.
4. Possible side effects
Like all medicines, Pandemrix can cause side effects, although not everybody gets them.
- Allergic reactions may occur following vaccination, in rare cases leading to shock. Doctors are aware of this possibility and have emergency treatment available for use in such cases.
In the clinical studies with a similar vaccine, most side effects were mild in nature and short term. The side-effects are generally similar to those related to the seasonal flu vaccine.
- The frequency of possible side effects listed below is defined using the following convention:
a) Very common (affects more than 1 user in 10)
b) Common (affects 1 to 10 users in 100)
c) Uncommon (affects 1 to 10 users in 1,000)
d) Rare (affects 1 to 10 users in 10,000)
e) Very rare (affects less than 1 user in 10,000)
The side effects listed below have occurred with Pandemrix in clinical studies in adults, including the elderly and in children aged from 3-9 years:
Very common:
• Headache
• Tiredness
• Pain, redness, swelling or a hard lump at the injection site
• Fever
• Aching muscles, joint pain
Common:
• Warmth, itching or bruising at the injection site
• Increased sweating, shivering, flu-like symptoms
• Swollen glands in the neck, armpit or groin
Uncommon:
• Tingling or numbness of the hands or feet
• Sleepiness
• Dizziness
• Diarrhoea, vomiting, stomach pain, feeling sick
• Itching, rash
• Generally feeling unwell
• Sleeplessness
In children aged 3-9 years fever occurred more often when the adult dose (0.5 ml of vaccine) was given compared to administration of half the adult dose (0.25 ml of vaccine). Also fever occurred more often in children aged 6-9 years compared to the children aged 3-5 years.
These side effects usually disappear within 1-2 days without treatment. If they persist, CONSULT YOUR DOCTOR.
The side effects listed below have occurred in the days or weeks after vaccination with vaccines given routinely every year to prevent flu. These side effects may occur with Pandemrix.
Uncommon
• Generalised skin reactions including urticaria (hives)
Rare
• Allergic reactions leading to a dangerous decrease of blood pressure, which, if untreated, may lead to shock. Doctors are aware of this possibility and have emergency treatment available for use in such cases.
• Fits
• Severe stabbing or throbbing pain along one or more nerves
• Low blood platelet count which can result in bleeding or bruising
Very rare
• Vasculitis (inflammation of the blood vessels which can cause skin rashes, joint pain and kidney problems)
• Neurological disorders such as encephalomyelitis (inflammation of the central nervous system), neuritis (inflammation of nerves) and a type of paralysis known a Guillain-Barré Syndrome
If any of these side effects occur, please tell your doctor or nurse immediately.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor.
5. How to store Pandemrix
Keep out of the reach and sight of children.
Before the vaccine is mixed:
· Do not use the suspension and the emulsion after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
· Store in a refrigerator (2°C - 8°C).
· Store in the original package in order to protect from light.
· Do not freeze.
After the vaccine is mixed:
· After mixing, use the vaccine within 24 hours and do not store above 25°C.
· Medicines should not be disposed of via wastewater or household waste.
· Ask your pharmacist how to dispose of medicines no longer required.
· These measures will help to protect the environment.
6. Further information
What Pandemrix contains
1. Active substance:
Split influenza virus, inactivated, containing antigen* equivalent to:
A/California/7/2009 (H1N1)v-like strain (X-179A) 3.75 micrograms** per 0.5 ml dose
* propagated in eggs
** expressed in microgram haemagglutinin
This vaccine complies with the WHO recommendation and EU decision for the pandemic.
2. Adjuvant:
The vaccine contains an ‘adjuvant’ AS03 to stimulate a better response.
This adjuvant contains squalene (10.69 milligrams), DL-a-tocopherol
(11.86 milligrams) and polysorbate 80 (4.86 milligrams).
3. Other ingredients:
The other ingredients are: polysorbate 80, octoxynol 10, thiomersal, sodium chloride, disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, magnesium chloride, water for injections
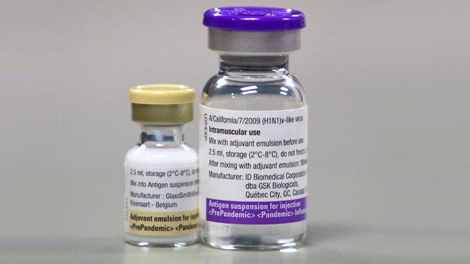
What Pandemrix looks like and contents of the pack ?
Suspension and emulsion for emulsion for injection.
The suspension is a colourless light opalescent liquid.
The emulsion is a whitish homogeneous liquid.
Prior to administration, the two components should be mixed. The mixed vaccine is a whitish emulsion.
One pack of Pandemrix consists of:
• One pack containing 50 vials of 2.5 ml suspension (antigen) for 10 doses
• Two packs containing 25 vials of 2.5 ml emulsion (adjuvant) for 10 doses
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder:
This leaflet was last approved in 09/2009.
Pandemrix has been authorised under “Exceptional Circumstances”.
The European Medicines Agency (EMEA) will regularly review any new information on the medicine and this package leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web site: http://www.emea.europa.eu/
Pandemrix is a registered trademark of the GlaxoSmithKline group of companies
©2009 GlaxoSmithKline group of companies
Articles
2356
Home Visit Service
Your Baby checkup
Is my child developing normally?
what are the vaccinations that he should have taken until now?
Generate a report for my baby.
what are the vaccinations that he should have taken until now?
Generate a report for my baby.
Birthdate *
Track Your Baby Vaccinations
Receive reminders by email for the Vaccination timing
Find Your Baby name
Visit our Clinics
Mohandessin
Address
View Map
21 Batal Ahmed Abdel Aziz St, 3rd floor
Telephones
01002195777
01000012400
0233048350
Beverly Hills
Address
View Map
Beverly Hills, Building 29 services, behind Super Market Al Mokhtar, floor 1.
Telephones
01000012900
0238576831
El Tagamo3
Address
View Map
Elegantry Mall, Unit 221
Telephones
01000012800
01000884592
Al Sheikh Zayed
Address
View Map
Al Sheikh Zayed - Entrance 2,Downtown Mall - In-front of Spectra ,First Floor - Clinic 113
Telephones
02- 38514031
01000608597
Please enter your e-mail


