Nov. 23, 2010
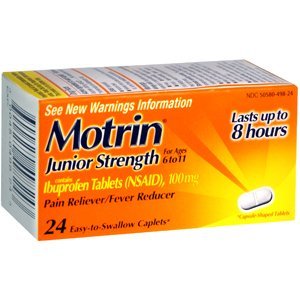
Johnson & Johnson says it has ordered a voluntary recall of about 4 million packages of Children’s Benadryl allergy tablets and 800,000 bottles of junior-strength Motrin caplets.
The company says the medicines are not dangerous and that consumers may keep taking the medications.
No Adverse Events Reported
Company spokeswoman declared that this is not a consumer level recall. She stresses that it is not being undertaken on the basis of adverse events, but because the company identified insufficiencies in the manufacturing process, after a review conducted as part of McNeil’s comprehensive action plan. The news release says there is no indication that the recalled products do not meet quality standards.
Products being recalled are Children’s Benadryl Allergy Fastmelt Tablets, cherry flavor, with a code number of 50580-347-18; Children’s Benadryl Allergy Fastmelt Tablets, grape flavor, code number 50580-348-18; and Junior Strength Motrin Caplets, 24 count, code number 50580-498-24. The tablets were distributed in the U.S., Canada, Puerto Rico, St. Thomas, St. Martin, Barbados, and Belize, the company says.
Source:http://children.webmd.com/news/20101123/benadryl-and-motrin-for-kids-recalled?ecd=wnl_nal_112310
Your Baby checkup
what are the vaccinations that he should have taken until now?
Generate a report for my baby.
Track Your Baby Vaccinations
Find Your Baby name
Mohandessin
01002195777
01000012400
0233048350
Beverly Hills
01000012900
0238576831
El Tagamo3
Al Sheikh Zayed
02- 38514031
01000608597



